Chiome Bioscience (Company note – 1Q update)

| Share price (6/17) | ¥130 | Dividend Yield (24/12 CE) | – % |
| 52weeks high/low | ¥218/111 | ROE(23/12 act) | -83.6 % |
| Avg Vol (3 month) | 436.7 thou shrs | Operating margin (TTM) | -176.6 % |
| Market Cap | ¥7.3 bn | Beta (5Y Monthly) | 1.26 |
| Enterprise Value | ¥5.6 bn | Shares Outstanding | 55.714 mn shrs |
| PER (24/12 CE) | – X | Listed market | TSE Growth |
| PBR (23/12 act) | 6.00 X |
| Click here for the PDF version of this page |
| PDF Version |
Drug Discovery and Development Business progressing well. Drug Discovery Support Business also in line with expectations.
◇ 1Q FY12/2024 results summary
Chiome Bioscience’s 1Q results for FY12/2024 were sales of 129 million yen (-23% YoY), an operating loss of 322 million yen, an ordinary loss of 303 million yen, and a net loss of 304 million yen.
Sales fell as transactions declined due to delays in the acceptance inspection of new projects and organisational changes within existing customers. Meanwhile, the losses below operating levels increased due to higher CMC (Chemistry, Manufacturing and Controls; chemistry, manufacturing, and quality control of active pharmaceutical ingredients and formulations) costs related mainly to CBA-1535 in research and development costs over the previous year.
The company’s full-year Drug Discovery Support Business sales forecast of 720 million yen (+6% YoY) remains unchanged. The increase in costs is mainly due to higher R&D costs related to the progress of clinical trials. Therefore, the profit/loss situation in 1Q is typical of a bio-venture company’s R&D activities, and there is no cause for concern, given that the company’s capital, cash, and deposits have been replenished through the exercise of stock acquisition rights.
◇ Pipeline and business development trends
No significant changes, but steady progress is being made.
For ADCT-701, an external clinical trial, the investigational entity has been transferred to the National Cancer Institute (NCI). NCI has filed an IND application for a Phase I trial in the US, and preparations are progressing toward starting a clinical trial in pediatric neuroendocrine cancer. In addition, the licensing agreement with ADC Therapeutics (ADCT) has been terminated, and the company has regained all rights to the anti-DLK-1 antibody.
In-house developed CBA-1205 has continued to receive SD (stable) evaluations for tumour reduction in melanoma patients enrolled in the first part of the Phase 1 study and has been administered for over 33 months. In the second part of the Phase 1 study, PR (partial response: tumour reduction of 30% or more) was confirmed in one patient with hepatocellular carcinoma. The manufacturing of additional investigational drugs for long-term treatment has been completed. The decision was made to tighten the selection criteria for patients enrolled in the trial and to extend the trial period. Patient screening is underway to analyse the scientific relationship between PR cases and treatment with the drug and verify the potential of the drug as a therapeutic agent.
In the first part of the Phase I study, CBA-1535, developed in-house, was administered as a single agent to patients with solid tumours. The drug’s safety and initial efficacy are evaluated while the volume gradually increases. So far, no safety concerns have been identified, and changes in blood biomarkers indicating T-cell activation, which is the concept of this antibody. The second half of the project is scheduled to start once this efficacy signal has been confirmed.
In other drug discovery projects, new out-licensing activities are underway for PFKR and PXLR, which are pre-clinical.
◇ The focus going forward
The main focus of attention will be the progress of clinical trials for CBA-1205 and CBA-1535, which are in phase I trials, and the success or failure of out-licensing these and ADCT-701, PCDC and other products. In particular, if the development stage of CBA-1205 and CBA-1535, which are in clinical trials, improves, the out-licensing potential will increase, leading to a single-year profit and an improvement in the cash flow structure. Therefore, more attention is likely to be paid to these products’ development and licensing activities than in the past.
◇ Summary of 1Q results for F12/2024
Chiome Bioscience (hereafter referred to as ‘the company’) reported sales of 129 million yen (-23% YoY), an operating loss of 322 million yen, a recurring loss of 303 million yen, and a net loss of 304 million yen in 1Q F12/2024.
Sales fell as transactions declined due to delays in the acceptance inspection of new projects and organisational changes within existing customers. On the other hand, the cost of CMC (Chemistry, Manufacturing and Controls; chemistry, manufacturing and quality control of active pharmaceutical ingredients and formulations) recorded for CBA-1535, mainly related to research and development costs, increased from the previous year, resulting in larger losses below the operating levels.
Regarding BS, at the end of March 2024, total assets amounted to 1,753 million yen, cash and deposits were 1,325 million yen (unchanged from end-December 2023), and short-term loans payable were 313 million yen (up 22 million yen). Net assets amounted to 1,247 million yen (up by 90 million yen), as shares were issued in connection with the exercise of subscription rights.
The company’s full-year Drug Discovery Support Business sales forecast of 720 million yen (+6% YoY) remains unchanged. The increase in costs is mainly due to higher R&D costs related to the progress of clinical trials. The 1Q results are typical of a bio-venture’s R&D activities, and given that the company has replenished its capital and cash and cash equivalents through the exercise of stock acquisition rights, there are no particular concerns with the company’s performance.
As mentioned in our previous report, a basic agreement on outsourcing was signed with Takeda Pharmaceutical in the quarter under review for antibody production using the company’s ADLib® system. This agreement is expected to contribute to the company’s continuous performance.
| JPY, mn, % | Net sales | YoY % |
Oper. profit |
YoY % |
Ord. profit |
YoY % |
Profit ATOP |
YoY % |
EPS (¥) |
| 2019/12 | 447 | 110.3 | -1,401 | – | -1,410 | – | -1,403 | – | -44.61 |
| 2020/12 | 480 | 7.4 | -1,283 | – | -1,291 | – | -1,293 | – | -36.06 |
| 2021/12 | 712 | 48.3 | -1,334 | – | -1,329 | – | -1,479 | – | -36.74 |
| 2022/12 | 630 | -11.5 | -1,258 | – | -1,243 | – | -1,242 | – | -28.26 |
| 2023/12 | 682 | 8.2 | -1,205 | – | -1,217 | – | -1,220 | – | -24.62 |
| 2023/12 (CE) | – | – | – | – | – | – | – | – | – |
| 2023/12 1Q | 169 | 31.8 | -225 | – | -227 | – | -227 | – | -4.70 |
| 2024/12 1Q | 129 | -23.5 | -322 | – | -303 | – | -304 | – | -5.60 |
Note: The company discloses only the estimates for the Drug Discovery Support business (sales of 720 million yen), as it is difficult to make reasonable forecasts for the Drug Discovery and Development business.
Drug Discovery Support Business clients
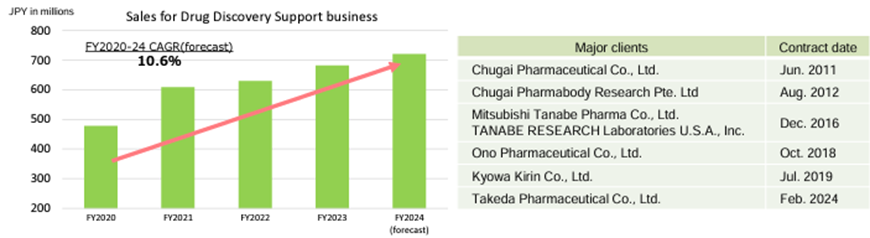
Source: Supplementary financial data for the 1Q FY12/2024 (dated 14 May, 2024)
◇Trends in the pipeline and business development
There have been no significant changes, but steady progress is being made.
For ADCT-701, for which external clinical trials are being conducted, the investigational entity has been transferred to the National Cancer Institute (NCI), which has filed an IND application for a Phase I trial in the US. Preparations are progressing toward the start of clinical trials for paediatric neuroendocrine cancer. In addition, the licensing agreement with ADCT has been terminated, and the company has regained all rights to the anti-DLK-1 antibody.
In-house developed CBA-1205 has continued to receive SD (stable) evaluations for tumour reduction in melanoma patients enrolled in the first part of the Phase 1 study and has been administered for over 33 months. In the second part of the Phase 1 study, PR (partial response: tumour reduction of 30% or more) was confirmed in one patient with hepatocellular carcinoma. The manufacturing of additional investigational drugs for long-term treatment has been completed. The decision was made to tighten the selection criteria for patients enrolled in the trial and to extend the trial period. Patient screening is underway to analyse the scientific relationship between PR cases and treatment with the drug to verify the potential of the drug as a therapeutic agent.
In the first part of the Phase I study, CBA-1535, developed in-house, was administered as a single agent to patients with solid tumours. The drug’s safety and initial efficacy are evaluated while the volume gradually increases. So far, no safety concerns have been identified, and changes in blood biomarkers indicating T-cell activation, which is the concept of this antibody. The second half of the project is scheduled to start once this efficacy signal has been confirmed.
In other drug discovery projects, new out-licensing activities for PFKR and PXLR, which are in pre-clinical trials, are underway.
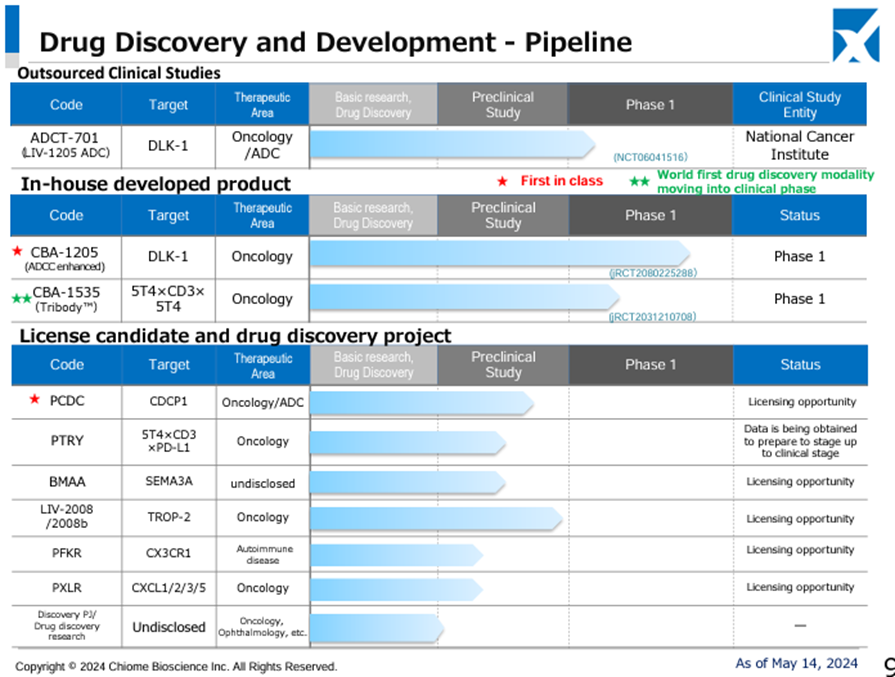
Source: Supplementary financial data for the 1Q FY12/2024 (dated 14 May, 2024)
◇ Progress in the pipeline: extending the plan to maximise the out-licensing value of CBA-1205
<In-house developed products>
*CBA-1205; Humanised anti-DLK-1 monoclonal antibody with enhanced ADCC activity. First-in-class. Second half of Phase I clinical trials. Positive signs were observed in Phase I clinical trials. Aimed at gaining multiple PR cases in hepatocellular carcinoma patients and maximising upfront licensing payments.
The first half part of the Phase 1 clinical trial of CBA-1205 was conducted at the National Cancer Centre in patients with solid tumours. The second part of the trial is being conducted in patients with hepatocellular carcinoma. The first part has already shown a high level of safety, and the melanoma patients enrolled in the study have continued to receive the drug for more than 33 months with SD (stable) evaluation with tumour shrinkage, and the drug is still being administered.
In addition, a partial response (PR: tumour reduction of 30% or more) was confirmed in one patient with hepatocellular carcinoma enrolled in the latter part of the study.
To verify the drug’s potential as a therapeutic agent, it was decided to tighten the selection criteria for patients enrolled in the trial and extend the trial period. To analyse the scientific relationship between PR cases and the administration of the drug, screening of patients enrolled in this part of the trial is currently being conducted. Correspondingly, additional manufacturing of the investigational drug has been completed, and the supply started in 4Q2023.
The second half of the Phase I clinical trial is scheduled for completion in 2025, and business alliance and out-licensing activities will proceed in parallel.
Licensing is expected to gain momentum if multiple PR cases in hepatocellular carcinoma patients are obtained.
*CBA-1535; Humanised anti-5T4 and anti-CD3 multispecificity antibody. World’s first drug discovery modality. First half of Phase I clinical trial underway, with second half to begin in 2024.
The company submitted a clinical trial plan notification to the PMDA in February 2022. At the end of June, it began Phase I clinical trials at the National Cancer Centre Hospital and Shizuoka Cancer Centre. Safety and efficacy signals were evaluated in patients with solid tumours in the first half of the Phase I clinical trial. The drug will be administered in stages, starting from a low volume to find the maximum dose that can be safely administered and to assess the initial drug effect signal.
To date, they have started to see changes in blood biomarkers indicating T-cell activation, which is the concept of this antibody. On the other hand, there are no safety-related data of development concern.
The second part will evaluate efficient drug efficacy in combination with cancer immunotherapy drugs. The second part will start after the efficacy signal is confirmed in the first part, with the second part beginning in 2024.
CBA-1535 is the world’s first clinical trial of TribodyTM and, if the concept is confirmed, will expand the applicability of TribodyTM to many cancer antigens. The combination of the number of binding targets and the number of moves to which they bind is expected to provide benefits in terms of patient quality of life and healthcare economics. It is expected to be more effective than conventional antibodies and to have multiple medicinal effects with only one dose of multiple drugs when administered in combination.
<Out-licensed products>
*ADCT-701 (LIV-1205); IND submission completed for Phase I clinical trials in the US.
The investigational entity has been changed from ADC Therapeutics of Switzerland (ADCT) to the National Cancer Institute (NCI). An Investigational New Drug (IND) application has been completed, and preparations are underway for the NCI to conduct a Phase I clinical trial for the treatment of paediatric neuroendocrine cancer.
This terminates the company’s licence agreement with ADCT and gives the company all rights to the anti-DLK-1 antibody. If development progresses after the NCI trial, the company will enter a new licence agreement with the pharmaceutical company.
<Out-licensing candidates>
*PCDC; First-in-class ADC targeting CDCP1, out-licensing activities continue.
PCDC is a humanised anti-CDCP1 antibody-drug conjugate (ADC) created by the company. Licensing activities target pharmaceutical companies with proprietary ADC technology that wish to use an antibody targeting CDCP1. Discussions are underway with several pharmaceutical companies, focusing on the scientific aspects.
CDCP1 is expressed in a wide range of solid tumours, including cancer types resistant to standard therapies, making the drug potentially first-in-class.
*PTRY; humanised 5T4, anti-CD3 and anti-PD-L1 multi-specific antibodies; a Tribody™ antibody with the potential to add immune checkpoint inhibition to the T cell engager function of CBA-1535.
Early evaluations in animal models have shown strong anti-tumour effects. Results of joint research on cancer immunotherapy conducted with the Italian public research institute Ceinge-Biotechnologie Avanzate in the Journal of Experimental & Clinical Cancer Research, an international journal. A patent application has been completed for the results obtained through this collaboration. In vivo efficacy data in a lung cancer model have confirmed that it exerts a strong tumour growth inhibitory effect.
The company focuses on research and development and exploring early licensing opportunities.
*PFKR ; a humanised anti-CX3CR1; antibody that targets CX3CR1, a GPCR, and is a new out-licensing candidate in the area of autoimmune CNS, which the company is investigating in collaboration with the National Institute of Neurology and Psychiatry. A patent application with secondary progressive multiple sclerosis (SPMS) and other diseases as potential indications has been completed. The number of patients with multiple sclerosis is estimated to be around 7,000 in Japan and more than 3 million worldwide. Licensing activities are underway.
*PXLR; humanised anti-CXCL1/2/3/5 antibody; administration of PXLR antibodies is expected to reduce immunosuppressive cells, overcome drug resistance and inhibit cancer recurrence. Intended for solid tumours (e.g. gastric, breast and ovarian cancer). A new out-licensing candidate that the Company has been researching in collaboration with Osaka Public University. A patent application has been completed. Licensing activities are underway.
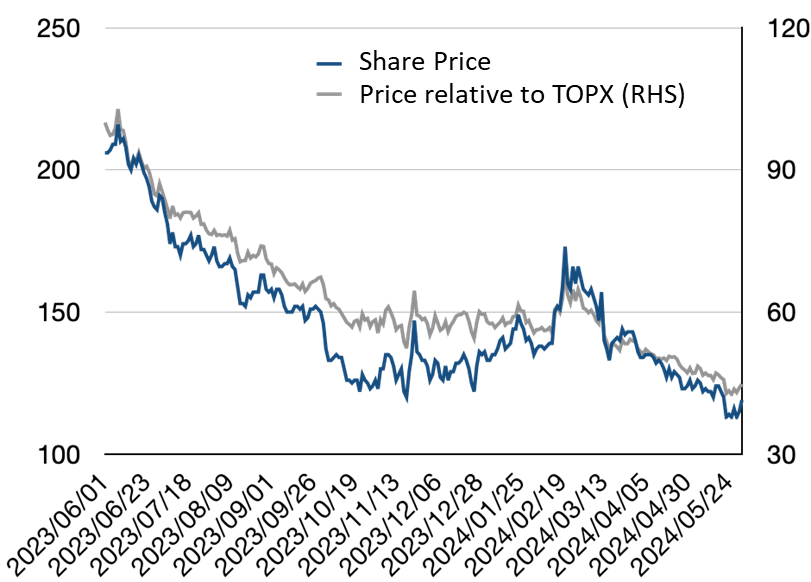
◇ Share price trends and the focus going forward: watch the progress of clinical trials for CBA-1205, CBA-1535, etc., and the out-licensing of PCDC.
The company’s share price has shown the following trends over the past year: Although it has been gradually declining, there are signs of a slight halt.
One reason for the gradual decline in share prices is the company’s current profit structure. Although the Drug Discovery Support Business is growing steadily, more is needed to cover R&D costs fully. As a result, the company has continued to raise the necessary funds by issuing shares through subscription rights and other means, and investors have become aware of this.
However, it is worth noting that, as we saw earlier, steady progress is being made in expanding the pipeline, particularly in CBA-1205, where there are promising drug cases, given that CBA-1205 and CBA-1535 are scheduled to complete the late phase 1 part of their clinical trials by the end of 2025, From a schedule perspective, investors are becoming aware of the upside potential from licensing-out. If the company achieves licensing-out, investor sentiment will improve as the company will be able to generate a profit in a single year from the upfront payment income and gain R&D resources that are not from share warrants.
The share price trend, which has recently stopped falling, suggests that investors know this upside. The progress of the company’s pipeline and licensing activities over the next 1-2 years will likely attract further attention.
Financial data
2021/12 |
2022/12 |
2023/12 |
2024/12 |
|||||||||||||
1Q |
2Q |
3Q |
4Q |
1Q |
2Q |
3Q |
4Q |
1Q |
2Q |
3Q |
4Q |
1Q |
2Q |
3Q |
4Q |
|
[Statements of income] |
||||||||||||||||
Net sales |
246 |
139 |
157 |
171 |
128 |
149 |
156 |
197 |
169 |
189 |
165 |
159 |
129 |
|||
Drug Discovery and DevelopmentBusiness |
103 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|||
Drug Discovery Support Business |
143 |
138 |
157 |
171 |
128 |
149 |
156 |
197 |
169 |
189 |
165 |
159 |
129 |
|||
Cost of sales |
64 |
62 |
78 |
86 |
57 |
69 |
72 |
83 |
73 |
76 |
67 |
68 |
72 |
|||
Gross profit |
182 |
77 |
79 |
84 |
70 |
80 |
84 |
114 |
95 |
112 |
98 |
94 |
56 |
|||
SG&A expenses |
337 |
337 |
515 |
568 |
557 |
373 |
344 |
334 |
321 |
545 |
344 |
394 |
379 |
|||
R&D expenses |
216 |
243 |
401 |
451 |
446 |
245 |
225 |
219 |
193 |
408 |
202 |
249 |
246 |
|||
Operating profit |
-155 |
-260 |
-436 |
-483 |
-486 |
-292 |
-260 |
-220 |
-225 |
-433 |
-246 |
-301 |
–322 |
|||
Non-operating income |
7 |
0 |
2 |
4 |
0 |
16 |
0 |
5 |
0 |
0 |
1 |
0 |
21 |
|||
Non-operating expenses |
1 |
0 |
1 |
6 |
4 |
1 |
1 |
-1 |
1 |
1 |
9 |
2 |
1 |
|||
Ordinary profit |
-150 |
-259 |
-434 |
-486 |
-491 |
-278 |
-261 |
-214 |
-227 |
-434 |
-254 |
-302 |
-303 |
|||
Extraordinary income |
0 |
6 |
0 |
1 |
0 |
1 |
0 |
0 |
||||||||
Extraordinary expenses |
0 |
0 |
0 |
|||||||||||||
Loss before income taxes |
-149 |
-247 |
-433 |
-636 |
-491 |
-278 |
-255 |
-214 |
-226 |
-434 |
-254 |
-301 |
-302 |
|||
Total income taxes |
11 |
1 |
1 |
0 |
1 |
2 |
1 |
1 |
1 |
1 |
1 |
2 |
1 |
|||
Net income |
-161 |
-248 |
-434 |
-637 |
-492 |
-279 |
-257 |
-215 |
-227 |
-435 |
-254 |
-304 |
–304 |
|||
[Balance Sheets] |
||||||||||||||||
Current assets |
3,294 |
3,088 |
2,675 |
2,216 |
2,005 |
1,792 |
1,955 |
2,092 |
1,964 |
1,566 |
1,633 |
1,629 |
1,621 |
|||
Cash and deposits |
2,580 |
2,302 |
2,071 |
1,790 |
1,744 |
1,471 |
1,592 |
1,727 |
1,566 |
1,245 |
1,341 |
1,326 |
1,325 |
|||
Non-current assets |
244 |
241 |
274 |
122 |
121 |
128 |
126 |
123 |
120 |
118 |
119 |
122 |
132 |
|||
Tangible assets |
6 |
6 |
4 |
4 |
3 |
3 |
2 |
2 |
2 |
1 |
1 |
1 |
0 |
|||
Investments and other assets |
237 |
235 |
269 |
118 |
117 |
124 |
122 |
120 |
118 |
117 |
117 |
121 |
132 |
|||
Total assets |
3,537 |
3,329 |
2,950 |
2,339 |
2,126 |
1,920 |
2,081 |
2,215 |
2,085 |
1,685 |
1,753 |
1,751 |
1,753 |
|||
Current liabilities |
378 |
428 |
468 |
392 |
419 |
390 |
376 |
370 |
469 |
486 |
4887 |
539 |
451 |
|||
Short-term borrowings |
180 |
190 |
199 |
183 |
183 |
188 |
188 |
184 |
304 |
298 |
316 |
291 |
313 |
|||
Non-current liabilities |
42 |
42 |
53 |
53 |
53 |
54 |
54 |
54 |
54 |
54 |
54 |
55 |
54 |
|||
Total liabilities |
420 |
470 |
522 |
446 |
473 |
444 |
431 |
424 |
523 |
540 |
542 |
594 |
506 |
|||
Total net assets |
3,118 |
2,859 |
2,428 |
1,893 |
1,653 |
1,476 |
1,650 |
1,790 |
1,562 |
1,144 |
1,211 |
1,157 |
1,247 |
|||
Total shareholders’ equity |
3,118 |
2,859 |
2,428 |
1,857 |
1,621 |
1,445 |
1,631 |
1,777 |
1,549 |
1,132 |
1,189 |
1,139 |
1,234 |
|||
Capital stock |
1,471 |
1,471 |
1,472 |
1,515 |
1,642 |
1,695 |
1,916 |
2,097 |
2,097 |
2,106 |
2,262 |
2,388 |
2,587 |
|||
Legal capital reserve |
3,071 |
3,071 |
3,072 |
3,115 |
3,242 |
3,295 |
3,516 |
3,696 |
3,696 |
3,706 |
3,861 |
3,988 |
4,187 |
|||
Retained earnings |
-1,455 |
-1,703 |
-2,136 |
-2,773 |
-3,262 |
-3,544 |
-3,801 |
-4,016 |
-4,244 |
-4,679 |
-4,934 |
-5,236 |
-5,540 |
|||
Subscription rights to shares |
30 |
19 |
19 |
35 |
31 |
30 |
18 |
13 |
12 |
12 |
22 |
18 |
12 |
|||
Total liabilities and net assets |
3,537 |
3,329 |
2,950 |
2,339 |
2,126 |
1,920 |
2,081 |
2,215 |
2,085 |
1,685 |
1,753 |
1,751 |
1,753 |
|||
[Statements of cash flows] |
||||||||||||||||
Cash flow from operating activities |
-560 |
-1,131 |
-660 |
-1,191 |
-595 |
-1,069 |
||||||||||
Loss before income taxes |
-396 |
-1,466 |
-768 |
-1,237 |
-661 |
-1,215 |
||||||||||
Cash flow from investing activities |
– |
-35 |
– |
– |
0 |
0 |
||||||||||
Purchase of investment securities |
– |
– |
– |
– |
– |
– |
||||||||||
Cash flow from financing activities |
176 |
271 |
341 |
1,127 |
113 |
667 |
||||||||||
Proceeds from issuance of common shares |
166 |
253 |
336 |
1,126 |
– |
555 |
||||||||||
Net increase in cash and cash equiv. |
-384 |
-895 |
-319 |
-63 |
-481 |
-402 |
||||||||||
Cash and cash equiv. at beginning of period |
2,686 |
2,686 |
1,790 |
1,790 |
1,727 |
1,727 |
||||||||||
Cash and cash equiv. at end of period |
2,301 |
1,790 |
1,471 |
1,727 |
1,245 |
1,326 |
||||||||||
Note) For the cash flow statement, Q2 is the cumulative of Q1 to Q2, and Q4 is the cumulative of Q1 to Q4. Therefore, the beginning balance will be the beginning balance of Q4 for both Q2 and Q4.
Source: Omega Investment from Company materials.
