Sosei Group

Sosei Group (4565)
| Securities Code |
| TYO:4565 |
| Market Capitalization |
| 118,365 million yen |
| Company Site |
| https://soseiheptares.com/home?ctry=jp |
| IR Contact |
| ir@soseiheptares.com |
Scoreboard
| Discovery | Development | Capital | Impact | Overall | Quartile |
| 3.4 | 4.0 | 4.4 | 3.8 | 4.0 | 1 |
Company Profile
Sosei Heptares is an international biopharmaceutical company with a strong focus on cutting-edge drug discovery using unique tech-based drug discovery platform. We aim to achieve stable revenue growth without substantial investments by concentrating our R&D resources on drug discovery, licensing drug candidates to major global pharmaceutical companies, and gaining milestone and royalty revenues (up to a mid-teen percent of net sales).
Core Technology
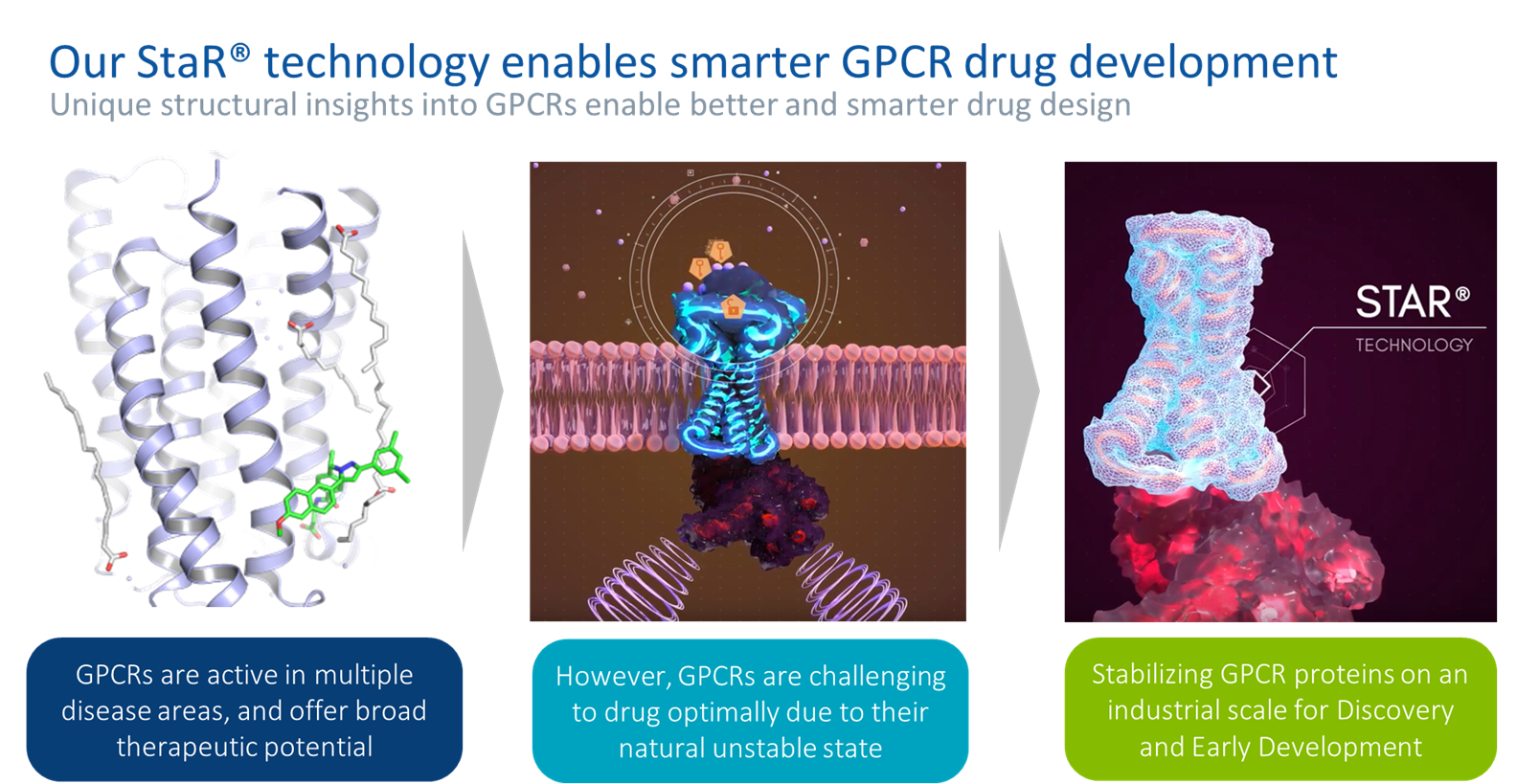
StaR®
StaR® (Stabilized Receptor) technology forms the backbone of Sosei Heptares’ integrated SBDD platform that enables us to “unlock” the potential of GPCRs through an advanced understanding of their structure. Our StaR® technology allows us to stabilize a GPCR by engineering a small number of single point mutations outside of the ligand-binding site such that they retain their organized structure even after they are removed from the cell membrane. The resulting stabilized proteins (StaR® proteins) are much more robust than the corresponding “wild-type,” or unmutated, proteins. These StaR® proteins are more readily purified and subjected to a variety of hit discovery and biophysical approaches. For example, StaR® proteins enable crystallization for detailed X-ray (or other) structure determination, which facilitates the design of innovative medicines with better safety and efficacy profiles and lower preclinical and clinical attrition rates compared to wild-type proteins. StaR® technology also enables the production of stabilized proteins that can be used for biologics discovery, either via in vitro phage screening, or for in vivo immunization.
Active pipeline
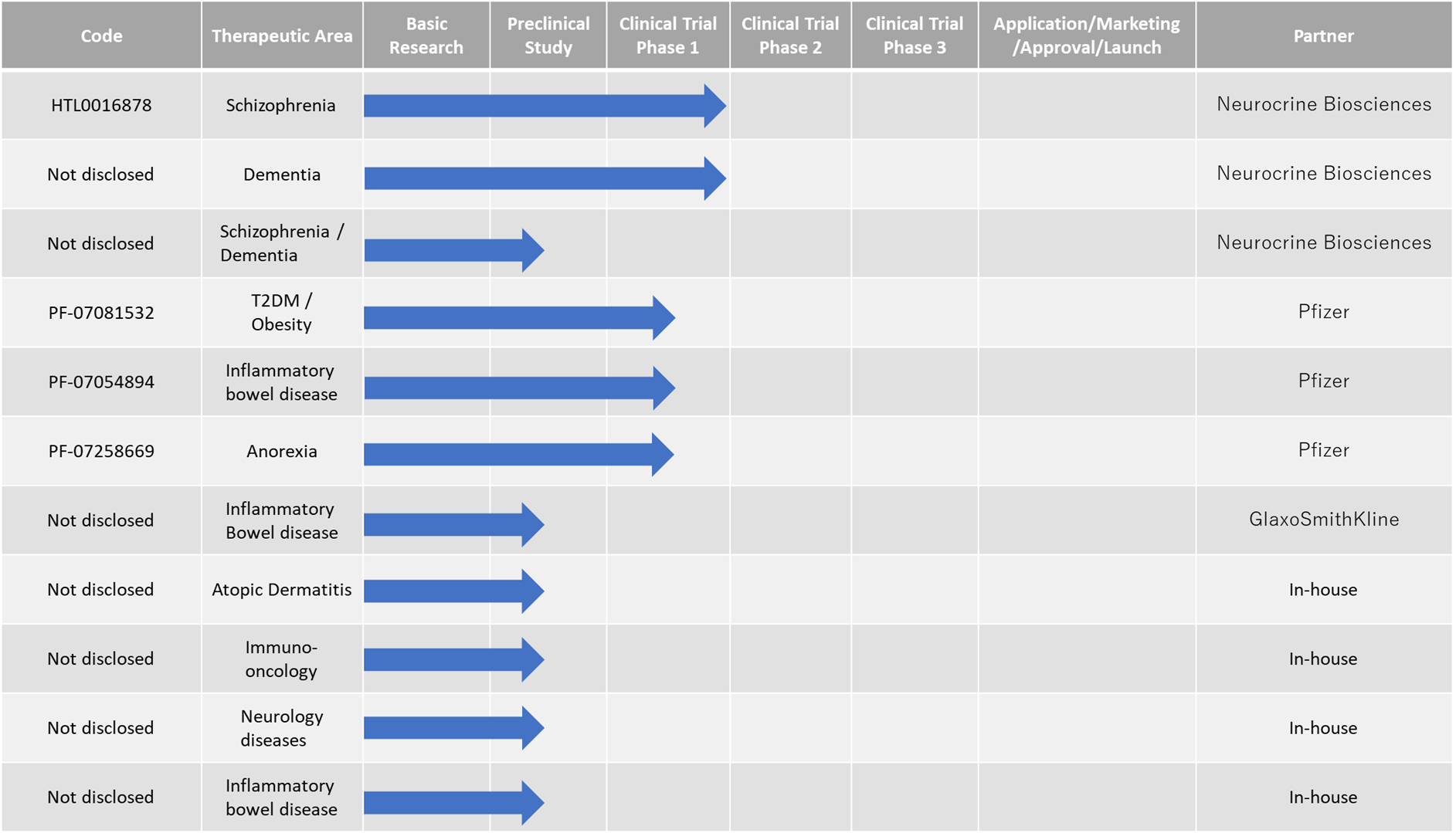
| HTL0016878/muscarinic M4 agonist | Partner Neurocrine plans to start a Ph2 trial in 2022. |
| Muscarinic M1 agonist | Partner Neurocrine plans to start Ph1 trials in 2023. |
| Muscarinic M1/M4 agonist | Partner Neurocrine plans to start Ph1 trials in 2023. |
| PF-07081532/GLP-1 agonist | Current Ph1 trial by partner Pfizer will end in 2022. May advance to the next stage of development. |
| PF-07054894/CCR6 antagonist | Current Ph1 trial at partner Pfizer will be over in 2022. May advance to the next stage of development. |
| PF-07258669/MC4 antagonist | Current Ph1 trial at partner Pfizer will finish in 2022. May advance to the next stage of development. |
